IN a major advancement in nanomedicine, Arizona State University scientists, in collaboration with researchers from the National Center for Nanoscience and Technology (NCNST) of the Chinese Academy of Sciences, have successfully programmed nanorobots to shrink tumors by cutting off their blood supply.
“We have developed the first fully autonomous, DNA robotic system for a very precise drug
design and targeted cancer therapy,” said Hao Yan, director of the ASU Biodesign Institute’s Center for Molecular Design and Biomimetics and the Milton Glick Professor in the School of Molecular Sciences.
“Moreover, this technology is a strategy that can be used for many types of cancer, since all solid tumor-feeding blood vessels are essentially the same,” Yan said.
The successful demonstration of the technology, the first-of-itskind study in mammals utilising breast-cancer, melanoma, ovarian and lung-cancer mouse models, was published in the journal Nature Biotechnology.
Seek and destroy
Yan is an expert in the field of DNA origami, which in the past two
decades has developed atomicscale manufacturing to build more and more complex structures.
The bricks to build their structures come from DNA, which can self-fold into all sorts of shapes and sizes — all at a scale 1,000 Natural tooth repair method using Alzheimer’s drug
Illustration of a fetal lamb inside the “biobag” system. PIX/ The Children’s Hospital of
Philadelphia (CHOP). times smaller than the width of a human hair — in the hopes of one day revolutionising computing, electronics and medicine.
Nanomedicine is a new branch of medicine that seeks to combine the promise of nanotechnology to open up entirely new avenues for treatments, such as making
minuscule, molecule-sized nanoparticles to diagnose and treat difficult diseases, especially
cancer.
Until now, the challenge of advancing nanomedicine has been difficult because scientists wanted to design, build and carefully control nanorobots to actively seek and destroy cancerous tumors — while not harming any healthy cells.
The international team of researchers overcame this problem by using a seemingly simple
strategy to very selectively seek and starve out a tumor.
This work was initiated about five years ago. The NCNST researchers first wanted to specifically cut off tumor blood supply by inducing blood coagulation with high therapeutic efficacy and safety profiles in multiple solid tumors using DNA-based nanocarriers. Yan’s expertise has upgraded the nanomedicine design to be a fully programmable robotic system, able to perform its mission entirely on its own.
“These nanorobots can be programmed to transport molecular payloads and cause on-site tumor blood-supply blockages, which can lead to tissue death and shrink the tumor,” said Baoquan Ding, a professor at the NCNST in Beijing.
Nanobots to the rescue
To perform their study, the scientists took advantage of a well-known mouse tumor model, where human cancer cells are injected into a mouse to induce aggressive tumor growth.
Once the tumor was growing, the nanorobots were deployed to come to the rescue.
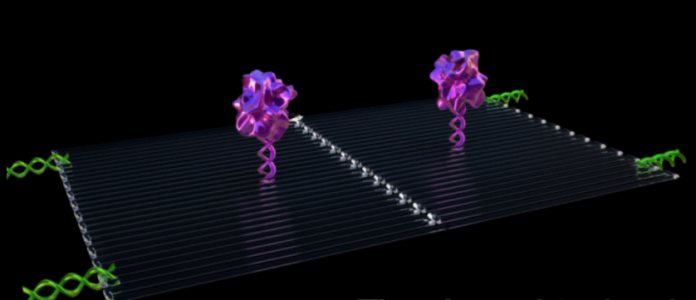
Each nanorobot is made from a flat, rectangular DNA origami sheet, 90 nanometers by 60 nanometers in size. A key blood-clotting enzyme, called thrombin, is attached to the
surface. Thrombin can block tumor blood flow by clotting the blood within the vessels that feed tumor growth, causing a sort of tumor mini heart attack and leading to tumor
tissue death.
First, an average of four thrombin molecules was attached to a flat DNA scaffold. Next, the flat sheet was folded in on itself like a sheet of paper into a circle to make a hollow tube. They were injected with an IV into a mouse, then traveled through the bloodstream, homing in on the tumors.
The key to programming a nanorobot that attacks only a cancer cell was to include a special payload on its surface, called a DNA aptamer. The DNA aptamer could specifically
target a protein, called nucleolin, that is made in high amounts only on the surface of tumor endothelial cells — and not found on the surface of healthy cells.
Once bound to the tumor blood vessel surface, the nanorobot was programmed, like the notorious Trojan horse, to deliver its unsuspecting drug cargo into the very heart of
the tumor, exposing the thrombin.
The nanorobots worked fast, congregating in large numbers to quickly surround the tumor just hours after injection. — Arizona State University