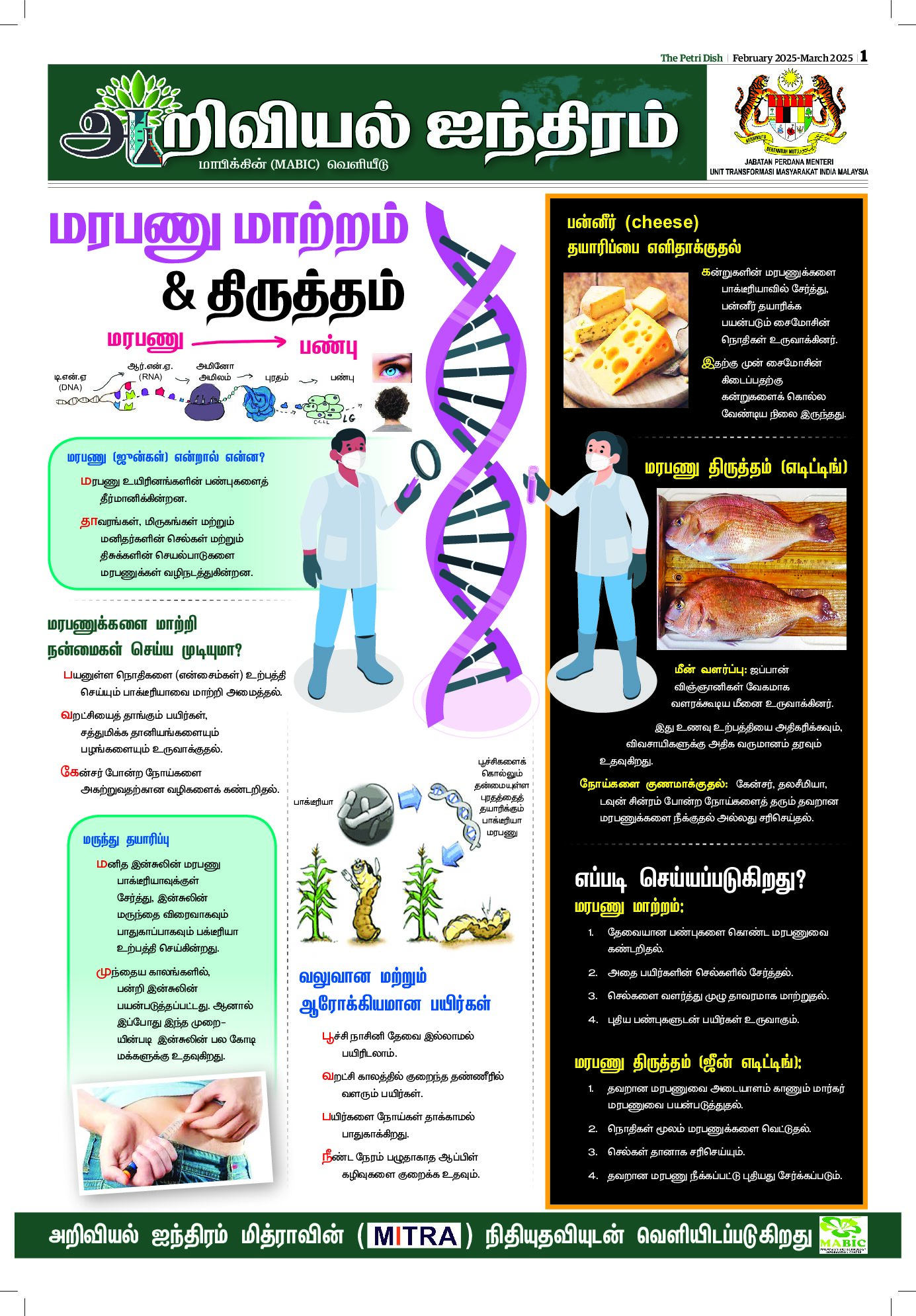
BY DR SADEQUR RAHMAN
AT the centre of all our lives is the necessity of getting enough food, which is more imperative than ever. Our population is almost eight billion and space on our planet is limited. How will we manage to avoid famines and social chaos?
It may seem a hopeless task, given all the global crises but we should be hopeful. We are an intelligent species and there are already tools available that could potentially let us avoid a food crisis. In several articles following this, I will look at how we may be able to avoid food shortages in a sustainable way.
In this article, I will discuss a powerful technique in our search for solutions. Not all of us may be familiar with it. Most of us know from experience that editing or correcting a piece of writing is much easier than rewriting from scratch.
Similarly correcting or improving instructions for how a plant copes with life—which is in the DNA of that plant – is much easier than coming up with completely new instructions.
Until a few years ago we did not have the ability to make these relatively small editorial changes to the DNA. We could certainly put small bits of DNA in the cells but we could not control where these new genes would reside.
This was like randomly putting in a sentence in an essay and hoping for an improved version. In the last few years though we have made massive gains in the ability to edit DNA to alter the blueprint of life to gain improved traits.
The capacity to edit DNA has come from observing bacteria and their struggles against viruses. Some bacteria can easily fight off viruses that have attacked them before. They do this by recognising the viral DNA. Scientists learnt from bacteria how to recognise DNA sequences more efficiently within the cell. Along with this ability to recognise specific sequences of DNA in the cell, we have also learnt how to edit it to provide beneficial traits.
Let’s take a simple example. Suppose we look at the cells in the petal of a flower. We know that in any cell, almost all the work is done by proteins. There are hundreds of proteins in the petal cells. Different DNA sequences in the cell have the instructions to make different proteins.
Imagine there is a particular protein in the petal cells which turns a white chemical into a red chemical. Let’s give this protein a made-up name—Colour protein. Now suppose the instructions for making Colour protein are only available for a short period of time and a small amount of Colour protein is made. As a result, a small amount of the white chemical is turned into the red chemical under normal circumstances. So the flower is pink.
Now comes the fun part. What if we edit the instructions so that Colour protein does not work? The flower then remains white. Now imagine we make a change in the other way. We edit the instructions so that the Colour Protein works ten times quicker. Now the flower becomes dark red as all the white chemical is turned into the red chemical.
In this simple example, we have made small changes to the pre-existing DNA to produce slightly altered instructions. These changes could have happened by chance over time but we made these changes take place rapidly. We have speeded up natural evolution.
How can such a technique be used in agriculture? A good example is a very recent announcement of producing tomatoes with high amounts of Vitamin D1. About one person in seven in the world suffers from Vitamin D deficiency. Such a deficiency can result in low resistance to infections and dementia and naturally there is a great demand for foods enriched with Vitamin D.
As a way of meeting this demand, gene editing has been used in the development of Vitamin D-enriched tomatoes. This is because tomatoes (and some other related plants) naturally produce a compound called 7-dehydrocholesterol (7DHC). If we consume 7DHC then this can be converted to Vitamin D by our bodies when we go out in the sun.
However, in the tomato fruit 7DHC is normally quickly used up by a protein to produce other complex chemicals for the plant. The situation is very similar to the conversion of the white chemical to a red chemical by the hypothetical Colour Protein.
Scientists edited the DNA that has instructions for making the protein that uses up 7DHC in the tomato fruit. They stopped it from working so it could not use up 7DHC and so there was a huge increase in the 7DHC in the fruit. It was estimated the amount of 7DHC in a gene-edited medium-sized tomato was the equivalent of that found in two eggs. Clearly, such approaches have the possibility of greatly benefitting the consumer, particularly if eggs are not consumed.
Let me know how you feel about gene editing. Should we edit our crops if it helps meet our needs? In the coming articles, we will discuss more extensively the tools and approaches available to us as we confront the challenges of feeding eight billion people in a sustainable way.
NOTE: The writer is a professor of plant genetics, School of Science, Monash University Malaysia.